The Abdominal Aortic Stent Grafts Pipeline Products Market is a rapidly evolving sector in the medical device industry, driven by the demand for minimally invasive solutions for abdominal aortic aneurysms (AAAs). With advancements in technology and a growing aging population, the market is expected to see significant growth in the coming years. This article provides an in-depth analysis of the market, covering key players, emerging trends, regulatory considerations, and more.
Understanding Abdominal Aortic Stent Grafts
Stent grafts are medical devices used to reinforce weakened or damaged blood vessels, particularly in the abdominal aorta. The abdominal aorta is the largest artery in the abdominal cavity, and it plays a crucial role in supplying blood to the lower body. When an aneurysm forms in the abdominal aorta, it can be life-threatening, leading to internal bleeding if it ruptures.
Abdominal aortic stent grafts are designed to provide a durable and minimally invasive solution to this problem. These devices consist of a metal stent and a fabric graft, which, when deployed, create a secure passage for blood flow, reducing the risk of rupture. The ability to treat AAAs with stent grafts has revolutionized vascular surgery, offering patients shorter recovery times and reduced risk compared to traditional open surgery.
Clinical Applications of Stent Grafts
The primary clinical application for abdominal aortic stent grafts is in the treatment of abdominal aortic aneurysms. These aneurysms are often asymptomatic, making them difficult to detect without imaging studies. Once identified, they require prompt intervention to prevent complications. Stent grafts provide a less invasive alternative to open surgical repair, allowing for endovascular aneurysm repair (EVAR).
EVAR involves the insertion of the stent graft through a small incision in the groin, using catheters to guide the device to the aneurysm site. The stent graft is then expanded to fit securely within the aorta, creating a new pathway for blood flow and excluding the aneurysm from the circulatory system. This approach has become the preferred method for treating AAAs due to its lower morbidity and shorter recovery times.
Emerging Technologies in Stent Grafts
The field of stent grafts is constantly evolving, with new technologies and innovations aimed at improving patient outcomes and expanding the range of treatable conditions. Some of the key emerging technologies in this market include:
Customizable stent grafts: These stent grafts can be tailored to fit the unique anatomy of individual patients, providing better outcomes and reducing the risk of complications.
Hybrid procedures: Combining endovascular and open surgical techniques, hybrid procedures offer flexibility in treating complex cases where traditional EVAR may not be suitable.
Advanced materials: The use of new materials in stent grafts, such as biocompatible polymers and nitinol, enhances durability and flexibility, allowing for greater customization and adaptability.
These emerging technologies are driving innovation in the market, enabling healthcare professionals to address a wider range of aneurysms with increased precision and safety.
Major Players in the Stent Grafts Market
The abdominal aortic stent grafts market is dominated by several major players, each contributing to the development and commercialization of these life-saving devices. Some of the leading companies in the market include:
Medtronic: A global leader in medical technology, Medtronic offers a range of stent graft products for abdominal aortic aneurysms, with a focus on innovation and patient safety.
Cook Medical: Cook Medical is known for its customizable stent grafts and hybrid procedures, providing solutions for complex cases and a variety of patient anatomies.
Gore Medical: With a strong reputation for quality and reliability, Gore Medical's stent grafts are designed with advanced materials, ensuring durability and optimal patient outcomes.
These major players invest heavily in research and development, continuously seeking to improve stent graft technology and expand their product offerings. Their contributions have significantly influenced the growth and evolution of the market.
Regulatory Considerations for Stent Grafts
Stent grafts, as medical devices, are subject to stringent regulatory requirements to ensure safety and efficacy. Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe play a critical role in overseeing the approval and monitoring of stent graft products.
Key regulatory considerations for stent grafts include:
Clinical trials: Manufacturers must conduct extensive clinical trials to demonstrate the safety and effectiveness of their stent graft products before receiving regulatory approval.
Post-market surveillance: After approval, manufacturers are required to conduct ongoing monitoring to ensure long-term safety and address any emerging issues.
Quality assurance: Stent graft manufacturers must adhere to strict quality assurance processes to maintain consistency and reliability in their products.
Understanding the regulatory landscape is essential for companies operating in this market, as compliance with these requirements is crucial for product approval and market success.
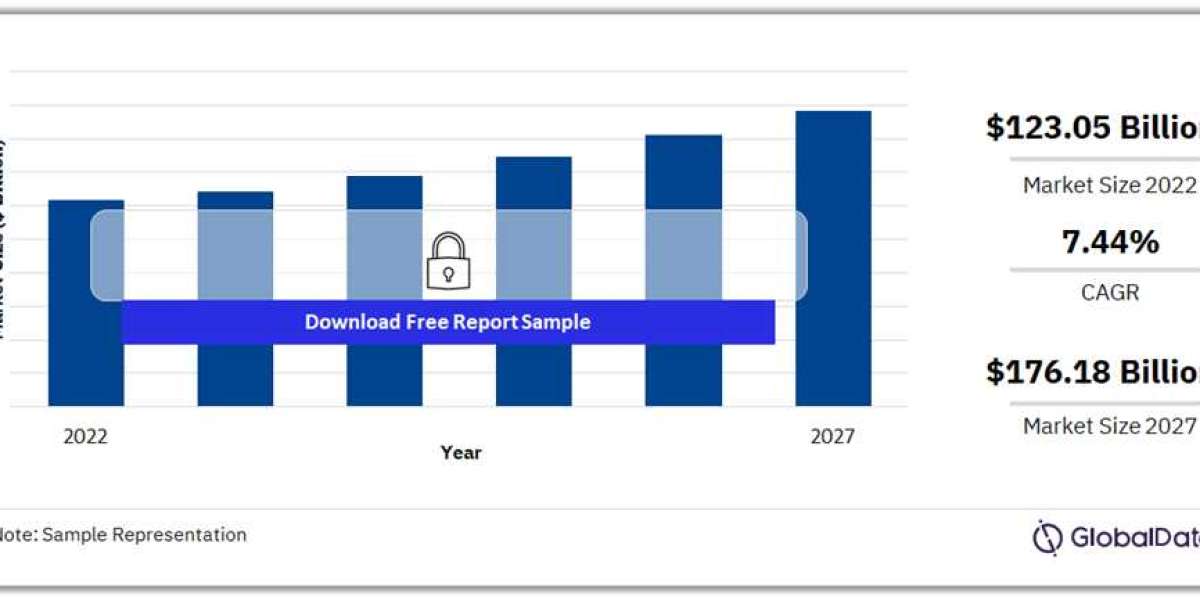
For more territory insights into the Abdominal Aortic Stent Grafts pipeline products market, download a free report sample